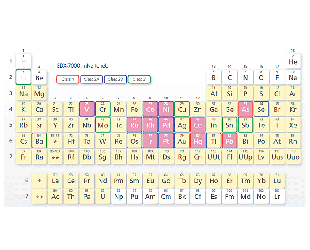
The pink grid squares indicate elements that can be accommodated by the Pharmaceuticals Impurities Analysis Method Package (optional).
Pharmaceutical Elemental Impurities Analysis System
Energy Dispersive X-ray Fluorescence Spectrometer
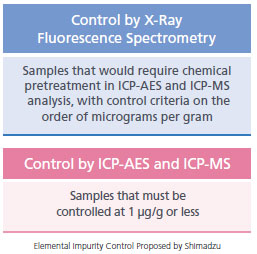
Control of Elemental Impurities in Pharmaceuticals In the pharmaceutical industry, the analysis of elemental impurities is necessary to ensure the safety of pharmaceuticals. In December 2014, the "Guideline for Elemental Impurities" (Q3D) was issued by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), consisting of representatives from Europe, the U.S. and Japan. In Japan, the "Guideline for Elemental Impurities in Drug Products" (PFSB/ELD Notification 0930 #4 from the Ministry of Health, Labour and Welfare) was issued, and will be applied to new drug products submitted for approval after April 1 2017. For 24 elements categorized in Class 1 to Class 3, residual quantities in pharmaceutical drug products must be controlled within permissible limits. Although ICP-AES and ICP-MS are used for precise analysis of elemental impurities, X-ray fluorescence spectrometers can be used as an alternative analysis method. This is because they can quantitatively and qualitatively analyze a variety of elements nondestructively, and without chemical pretreatment, unlike ICP-AES and ICP-MS systems. The X-ray fluorescence spectrometry has been adopted as a general method of analysis in the U.S Pharmacopeia and the European Pharmacopoeia. (USP<735>, Ph.Eur.2.2.37)
Element Classification (24 Elements)
- Class 1:Very toxic. Highly toxic in all administration routes.
- Class 2:Toxic, although it depends somewhat on the administration route.
- Class 2A:Assessment is required in all cases.
- Class 2AB:Assessment is required only when it is intentionally added in a process.
- Class 3:Toxicity is low in oral administration. Assessment is required for other administration routes.
PCEDX Pro Software

PCEDX Pro Software
Analysis is performed using PCEDX Pro software. Easy-to-use operations enable fully automatic measurements, so even novices can feel confident. This highly functional software supports many quantitative calculations, including the calibration curve method, the fundamental parameters (FP) method, the thin film FP method, and the background FP method (patented by Shimadzu).
Features
-
An optional vacuum measurement unit or helium purge unit is required to measure light elements (15P and below) with the EDX-7000.
The pink grid squares indicate elements that can be accommodated by the Pharmaceuticals Impurities Analysis Method Package (optional). -
The optical system is a bottom exposure type in which both the X-ray tube and the detector are built into the bottom of the instrument. Samples simply need to be placed in the measurement area (the part with the hole) within the sample chamber. The instrument is so designed that the shutter on the X-ray tube will not open...
-
When analyzing powder and liquid samples that cannot be positioned as is in the measurement area, place the sample in a sample cell covered with a special film for X-ray fluorescence. Then start analysis by irradiating the sample with X-rays through the film.
-
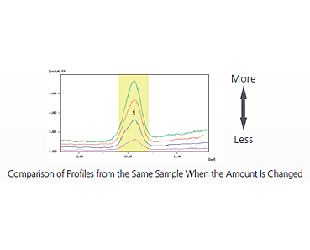
With the heavy elements included in organic substances, even fluorescent X-rays generated from deep in the sample penetrate the sample and reach the detector. As a result, the intensity of the fluorescent X-ray changes depending on differences in the sample amount (sample depth).