Analysis of the Reaction of Acrylate UV Curable Resin by UV Exposure / FTIR
The molecular structures of some polymers change over time when the polymers are mixed, heated, or exposed to light. Fourier transform infrared spectrophotometers (FTIR) are very effective at monitoring chemical reactions and changes because of their fast measurement capability. This article introduces an analysis of an acrylate UV curable resin that hardens in a relatively short time by radical polymerization due to UV ray exposure.
After a background measurement using a metallic plate as a reference, a thin layer of a sample was applied on the metallic plate while preventing peak saturation. A rapid scan measurement, obtaining 20 spectra every second, was started and UV irradiation was applied approximately 5 seconds later. Infrared spectra extracted per second are displayed below in 3D mode from the measurement results.

Time-Course Spectra 3D View of UV Curable Resin
The peak around 1635 cm-1 (C=C stretching vibration of vinyl group) and the peak around 810 cm-1 (CH out-of-plane bending vibration of vinyl group) rapidly dropped due to UV ray exposure.
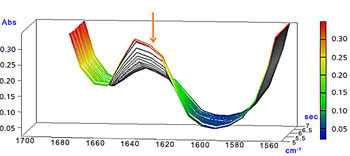
Enlarged View of Peak around 1635 cm-1
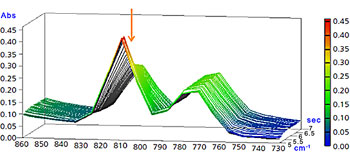
Enlarged View of Peak around 810 cm-1
The graphs show that the reaction rate reached over 50 % within 1 second after the UV ray was irradiated (5 seconds after the measurement started) and reached 80 % in approximately 5 seconds. The reaction then slowly progressed.
A time-course change of the reaction rate can be also calculated using rapid scan data.
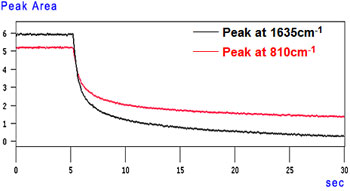
Time-Course Peak Area Graph
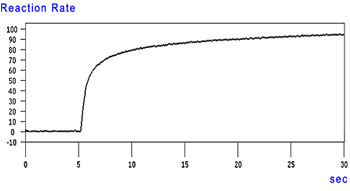
Time-Course Reaction Rate Change Graph
IRTracer-100
Fourier Transform Infrared Spectrophotometer

Featuring improvements in interferometer and detector design, the IRTracer-100 offers high sensitivity with a 60,000:1 S/N ratio. This sensitivity combined with the LabSolutions IR Contaminant Analysis Macro enables easier, quicker and more accurate analysis of small samples.


