Glycans

Glycosylation is one of the most common post-translational modification of proteins. The presence of the glycan moiety and its structural profile must be monitored, since it affects not only the effector function, but also ADME and immunogenicity.
Acquiring the glycan structure profile at an early stage of biotherapeutic development helps eliminate any molecules expressing high levels of unfavorable glycan species, e.g. fucosylation. This is taken into account during cell line selection to reduce longterm risk. MALDI-TOF MS methodology offers a simple, high-throughput option for this purpose.
Full Characterization and Monitoring

At later stages of biotherapeutics development, full characterization of glycan profile is required in order to define the reference glycan profile of a product for later QA/QC. High-resolution chromatography is needed for in-depth characterization, in order to separate and identify structural isomers that pose a health risk, e.g. alpha-1,3 galactose. Retention time gives reliable identification, and reproducibility of chromatography is the key driver for method selection.
i-Series Plus
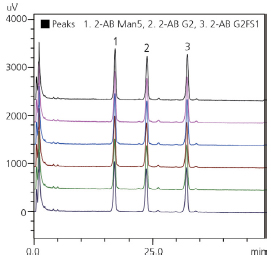
| Glycan standard | R.T. %RSD | Area %RSD |
|---|---|---|
| 2-AB Man5 | 0.273 | 0.743 |
| 2-AB G2 | 0.245 | 0.684 |
| 2-AB G2FSI | 0.196 | 0.589 |
Related Application Note
Glycans
- A Study on a Method for Evaluating Glycans in Biopharmaceuticals - Suppressing Peeling Reactions in Pretreatment for O-glycan Analysis -
- A Study on a Method for Evaluating Glycans in Biopharmaceuticals - Part 2
- Analysis of N-Linked Glycan using MALDImini™-1 Compact MALDI Digital Ion Trap Mass Spectrometer: Structural Analysis and Identification of Sialyl Linkage Isomers
Glycopeptides




