Simultaneous Analysis of Remdesivir and Metabolites in Human Plasma
Remdesivir (brand name: Veklury®), which was developed by Gilead Science (U.S.) for treatment of Ebola virus disease, is a prodrug having antiviral activity against single-strand RNA viruses. It is known to be partly metabolized to activated GS-441524, the main metabolite of remdesivir, in vivo1). In this article, we present the results of research into an analytical system that can analyze remdesivir and its metabolites simultaneously using LC/MS/MS, an analytical method that demonstrates outstanding selectivity (Fig. 1).
Analysis Conditions and Sample Pretreatment
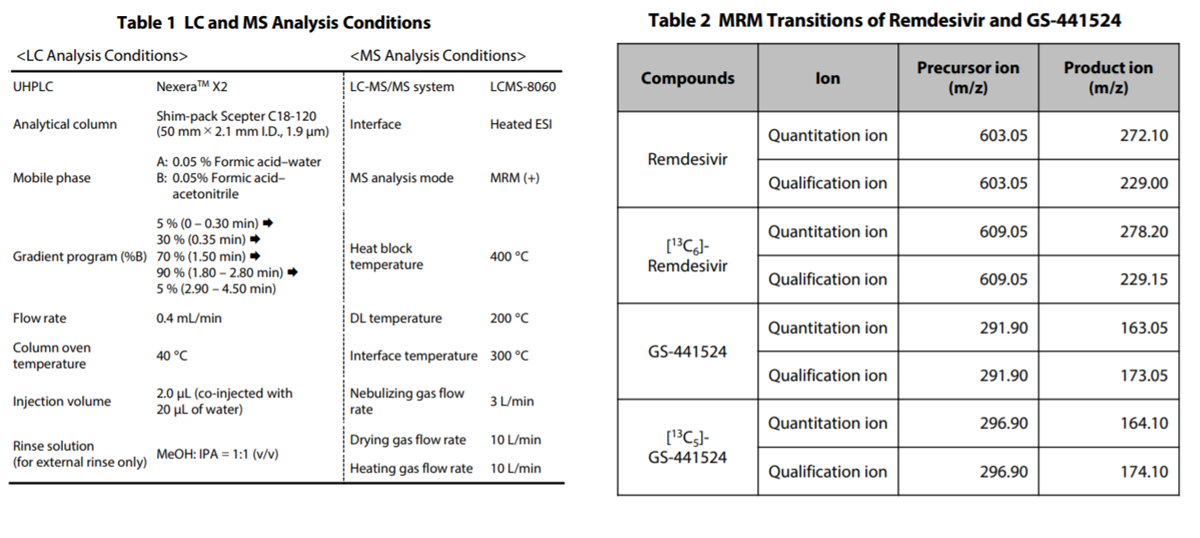
Remdesivir (P/N: C8799*) and GS-441524 (P/N: C8847*), as the target compounds, and [U-Ring-13C6]-remdesivir (P/N: C8845*) and [13C5]-GS-441524 (P/N: C8855*), as their stable isotopes, were purchased from Alsachim, one of the companies of the Shimadzu Group. [U-Ring-13C6]-remdesivir and [13C5]-GS- 441524 were used as materials of the internal standard. The structural formulas of remdesivir and GS-441524 are shown in Fig. 2. To commercially available human plasma treated with EDTA 2K, remdesivir and GS-441524 were added. Following this, the calibration curves were prepared. Analysis was performed using the LC and MS analysis conditions shown in Table 1 and the multiple reaction monitoring (MRM) data acquisition parameters shown in Table 2. Shim-pack ScepterTM C18-120 (50 mm×2.1 mm I.D., 1.9 μm) was used as the analytical column. Fig. 3 shows theMS chromatograms. Calibration was performed using 5 calibration points at concentrations of 100, 500, 1000, 2500 and 5000 ng/mL for remdesivir and 5 calibration points at concentrations of 5, 25, 50, 250 and 500 ng/mL for GS-441524 (n = 5 for each calibration point). [U-Ring-13C6]-remdesivir (2.5 μg/mL) and [13C5]-GS- 441524 (0.25 μg/mL) were mixed with methanol to be used as the internal standard (ISTD).
The samples are prepared in the following sequence. After 20 μL of 75% IPA, 50 μL of plasma, 10 μL of ISTD and 100 μL of acetonitrile were added and mixed well, the mixture was centrifuged. The supernatant obtained by centrifuging was transferred into an LC vial for analysis.
*Alsachim’sproduct numbers
![Structural Formulas of Remdesivir and GS-441524/Fig. 3 MS Chromatograms of Remdesivir, [U-Ring-13C6]- Remdesivir (Left) and GS-441524, [13C5]-GS-441524 (Right)](/sites/shimadzu.com.au/files/d7/ckeditor/an/industry/small_molecule_pharmaceutical/Preclinical/c218/f2-3.png)
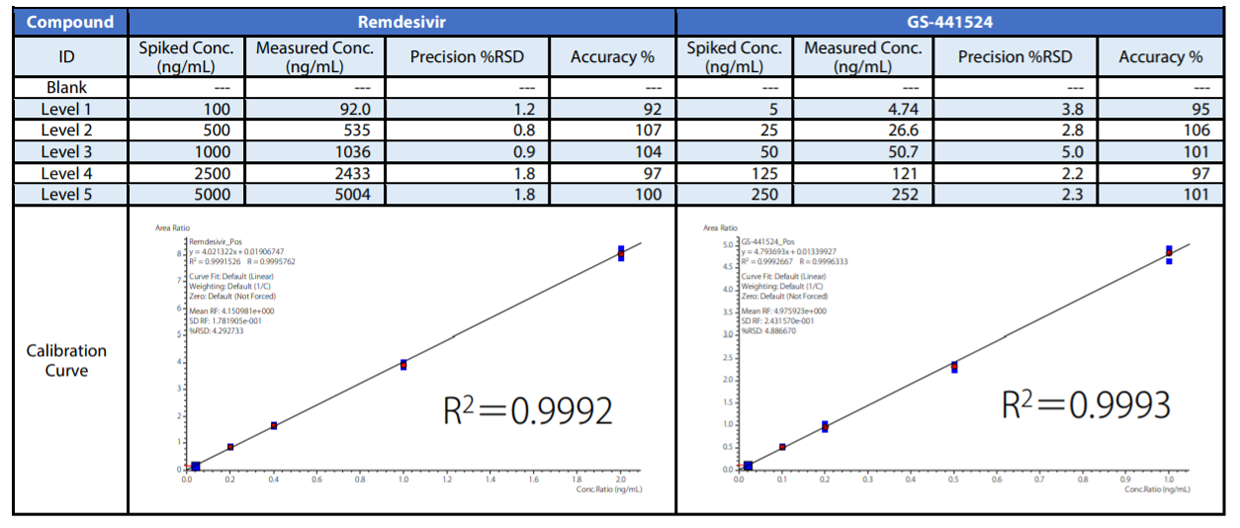
Preparation of Calibration Curves
Calibration curves of plasma-spiked samples are shown in Table 3. For Remdesivir and GS-441524, good linearity was obtained in the set calibration range. The precision (reproducibility) of remdesivir and GS-441524 in the entire concentration range, including the quantitative lower limit was %RSD 0.8 %-1.8 % and %RSD 2.2 %-5.0 %, respectively. Similarly, the accuracy of remdesivir and GS-441524 was 92.0 %-107 % and 94.8 %-106 %, indicating that the accuracy of both was within 100 ± 15 %.